Introduction
Duvelisib is a potent phosphoinositide-3-kinase delta (PI3K-δ) and gamma (PI3K-γ) inhibitor which has demonstrated efficacy in T-cell lymphomas (TCL) and low-grade B-cell Non-Hodgkin Lymphomas (B-NHL). It exerts its effect through inhibition of malignant cell proliferation and differentiation, as well as disruption of the tumor microenvironment. We aimed to evaluate the safety and efficacy of one year of duvelisib maintenance after consolidative autologous stem cell transplant (autoSCT) in patients with TCL and B-NHL.
Methods
Patients with TCL or B-NHL who received standard consolidative autoSCT began duvelisib maintenance after count recovery (approximately 30 days after transplant) and continued for 11 cycles (one year after transplant). Duvelisib started at 25 mg orally twice daily for two 28-day cycles (schedule 1), followed by 25 mg twice daily for 14 days on/ 14 days off (schedule 2) if the patient was in complete remission (CR) at day +100 or if intolerable toxicities developed. Patients in partial remission (PR) at day +100 continued schedule 1, and those with stable (SD) or progressive disease (PD) were taken off study.
Results
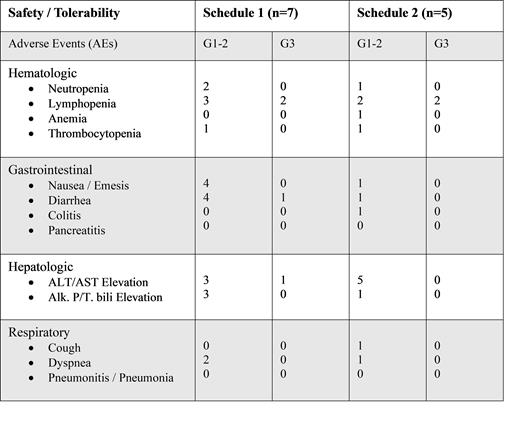
Twelve patients have been enrolled, starting in July 2020. Histologic subtypes were transformed follicular lymphoma (FL, n= 5), peripheral T-cell lymphoma (PTCL, n= 1), anaplastic large cell lymphoma (ALCL, n= 3), and angioimmunoblastic T-cell lymphoma (AITL, n= 3). Seven patients were male, median age was 62 (range, 25-71), and 10 patients had stage III/IV disease at diagnosis. Pre-transplant, 11 patients were in CR and 1 patient was in PR. Median follow-up was 23.7 months and median duration of duvelisib treatment was 8.7 months. Following enrollment of 7 patients, 2 patients developed grade 3 toxicities (elevated liver enzymes and diarrhea, respectively), prompting them to come off study. Patients were enrolled at schedule 2 thereafter, with subsequent improvement in tolerability. Most common adverse events (AEs) were hematologic, gastrointestinal, and hepatic (Table. 1). Grade 2-3 AEs probably related to treatment were seen in 6 patients: neutropenia (n= 2), lymphopenia (n= 1), diarrhea (n= 2), and elevated liver enzymes (AST/ALT) (n= 1). There were no grade 4-5 AEs and no deaths related to treatment. Permanent discontinuation of duvelisib due to grade 3 AEs occurred in 3 patients: elevated liver enzymes (AST/ALT), diarrhea, and lower extremity muscle weakness, respectively. The latter was deemed unlikely related to treatment and symptoms resolved upon follow-up. Of the 12 patients enrolled, 4 have relapsed (TCL, n= 2 and B-NHL, n= 2) and proceeded to additional treatment. All patients remain alive to date.
Conclusions
Duvelisib maintenance for up to one year after consolidative autoSCT in patients with TCL or B-NHL is safe and well tolerated at a dose schedule of 25 mg twice daily for 14 days on/ 14 days off, of 28-day cycles. Enrollment of patients undergoing consolidative autoSCT for TCL is ongoing to evaluate the efficacy of duvelisib maintenance.
Disclosures
Mehta-Shah:C4 Therapeutics: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Corvus Pharmaceuticals: Research Funding; Bristol Myers-Squibb: Research Funding; Genentech/Roche: Research Funding; Ono Pharmaceuticals: Consultancy; Karyopharm Therapeutics: Consultancy; Genentech: Consultancy; Secura Bio/Verastem: Consultancy, Research Funding; Janssen: Consultancy; Celgene: Research Funding; AstraZeneca: Consultancy, Research Funding; Kyowa Hakko: Consultancy; Innate Pharmaceuticals: Research Funding. Ghobadi:Genentech, Inc.: Research Funding; Wugen Inc: Consultancy; BMS: Consultancy; Kite/Gilead: Consultancy, Honoraria, Research Funding; CRISPR Therapeutics: Consultancy; Atara: Consultancy; Amgen: Consultancy, Research Funding. DiPersio:Magenta: Other: Ownership Investment, Patents & Royalties; WUGEN: Other: Ownership Investment, Patents & Royalties; Macrogenics: Consultancy, Research Funding; Washington University: Current Employment; RiverVest Venture Partners: Membership on an entity's Board of Directors or advisory committees; BiolineRx Ltd: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; NeoImmune Tech: Consultancy; Vertex: Consultancy; Sun Pharma Ltd.: Membership on an entity's Board of Directors or advisory committees; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; Amphivena Therapeutics: Research Funding. Cashen:Kite Pharma: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal